Ana Oña Blanco
Head of Service Contact
Biosecurity questionnaire / Cuestionario de bioseguridad/
Encuesta de satisfacción de los usuarios/ Users satisfaction survey
Equipment calendars
Confocal Stellaris
Confocal SP5
MicroFluor
Confocal SP8
Yoda (WF-TIRFM)
Confocal Stellaris 8 (STED 3X, FLIM, FCS y DLS) (STED 3X, FLIM, FCS y DLS)
Service Description
The unit provides state-of-the-art infrastructure for widefield, confocal laser scanning, nanoscopy and mesoscopy through STED, FLIM, FCS, DLS, TIRFM and SMLM techniques, covering most multicolour fluorescence imaging studies with fixed samples and live-cells. The equipment and services are available to all CNB personnel as well as to researchers from the public and private sectors. Technical staff offers assistance, training and advice about equipment use, available methodologies, image processing and, if required, quantification and analysis procedures. Cell culture slides, aliquots of secondary antibodies and probes with broad use in fluorescence microscopy applications are also provided. The facility’s equipment includes:
-
Leica STELLARIS 8 STED 3X multispectral confocal nano and mesoscopy system
- White laser with excitation range 440 to 790 nm and 405 nm diode.
- Two Power HyD S spectral detectors and two Power HyD X spectral detectors.
- STED 3X nanoscopy module with three depletion lines: 592, 660 and 775 nm.
- FLIM (Fluorecence Lifetime Imaging Microscopy) system.
- FCS (Fluorecence Correlation Spectroscopy) system.
- DLS (Digital Light Sheet) mesoscopy system.
- Integrated software module for real-time multidimensional super-resolution multidimensional image detection and processing (Lightning).
- Environmental control camera (Okolab) for time-lapse studies.
-
Leica TCS SP8 STED 3X multispectral confocal system
- White laser with excitation range 470 to 670 nm and 405 nm diode.
- Three HyD hybrid detectors with time gating.
- STED 3X nanoscopy module with two depletion lines: 592 and 660 nm
-
Leica STELLARIS 5 multispectral confocal system
- Four laser lines: 405, 488, 561 and 638 nm.
- Three Power HyD S spectral detectors.
- Integrated software module for real-time multidimensional super-resolution multidimensional image detection and processing (Lightning).
-
Leica TCS SP5 multispectral confocal system
- Seven laser lines: 405, 458, 476,488, 514, 561 and 633.
- Resonant scanner module for high-speed acquisition.
- Environmental control system for time-lapse studies.
-
Leica DMi8 S epifluorescence system
- Orca Flash 4.0 sCMOS camera (Hamamatsu).
- TIRFM (Total Internal Reflection Fluorescence Microscopy) module.
- High-power 405, 488, 561 and 638 nm lasers for Single-Molecule Localization Microscopy (PALM/STORM).
- Gemini W-View splitter (Hamamatsu) for simultaneous acquisition with green/red fluorochromes.
- Environmental control camera (Okolab) for time-lapse studies.
-
Leica DMI6000B epifluorescence microscope
- OrcaR2 monochrome digital camera.
- Environmental control system for time-lapse studies.
-
Three independent workstations with the software for image processing and analysis: LAS AF, LAS X, Huygens, Aivia, Imaris, MetaMorph, ImageJ/Fiji, Cell Profiler.
-
Auxiliary equipment for cell culture, preparation, processing and storage of samples: CO2 incubator, centrifuge, laminar flow hood, refrigerator and freezer.
-
- Multichannel confocal and/or transmission imaging of living or fixed samples (2D, 3D and 4D imaging).
- Specialized microscopy techniques: STED 3X, FLIM, FCS, DLS, TIRFM and SMLM.
- Multidimensional in vivo time-lapse experiments.
- High speed confocal microscopy.
- FRET, FRAP, photoactivation, photoswitching, excitation/emission lambda scan, reflection.
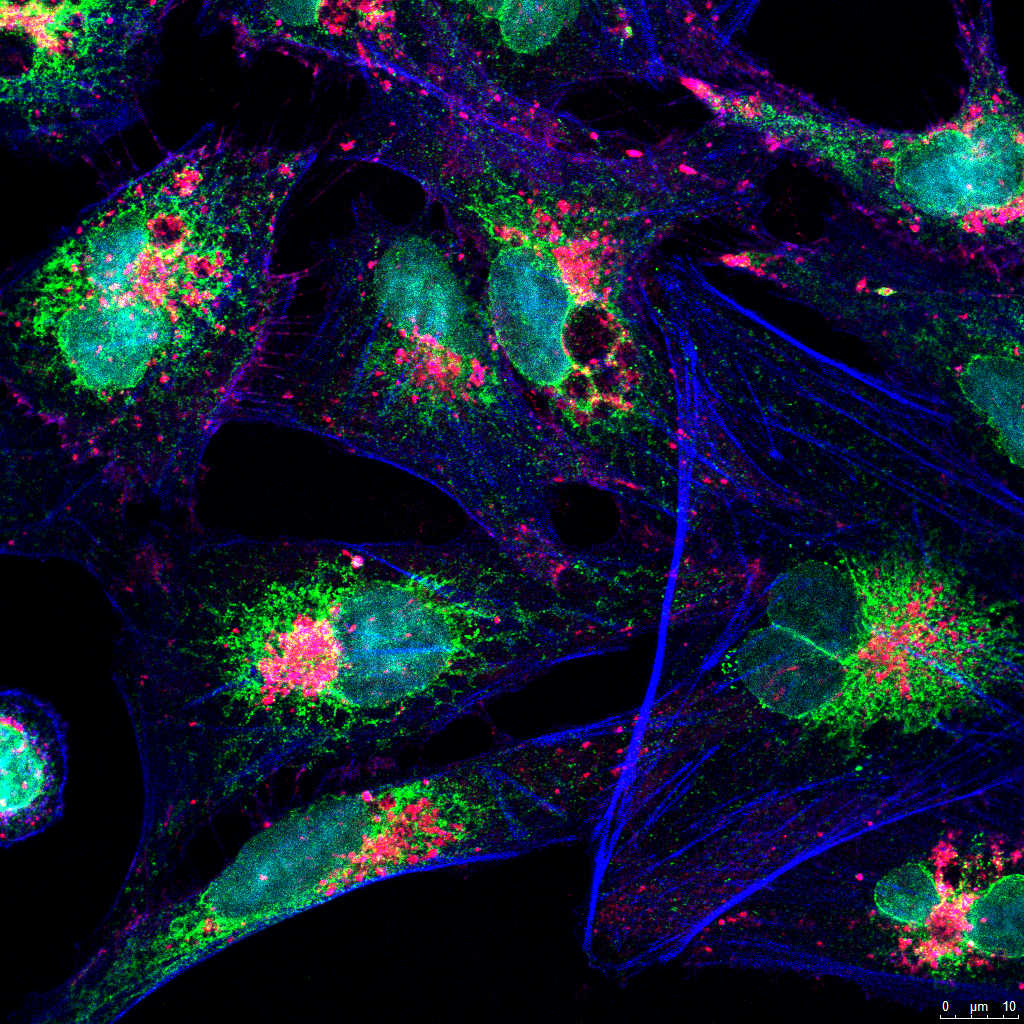
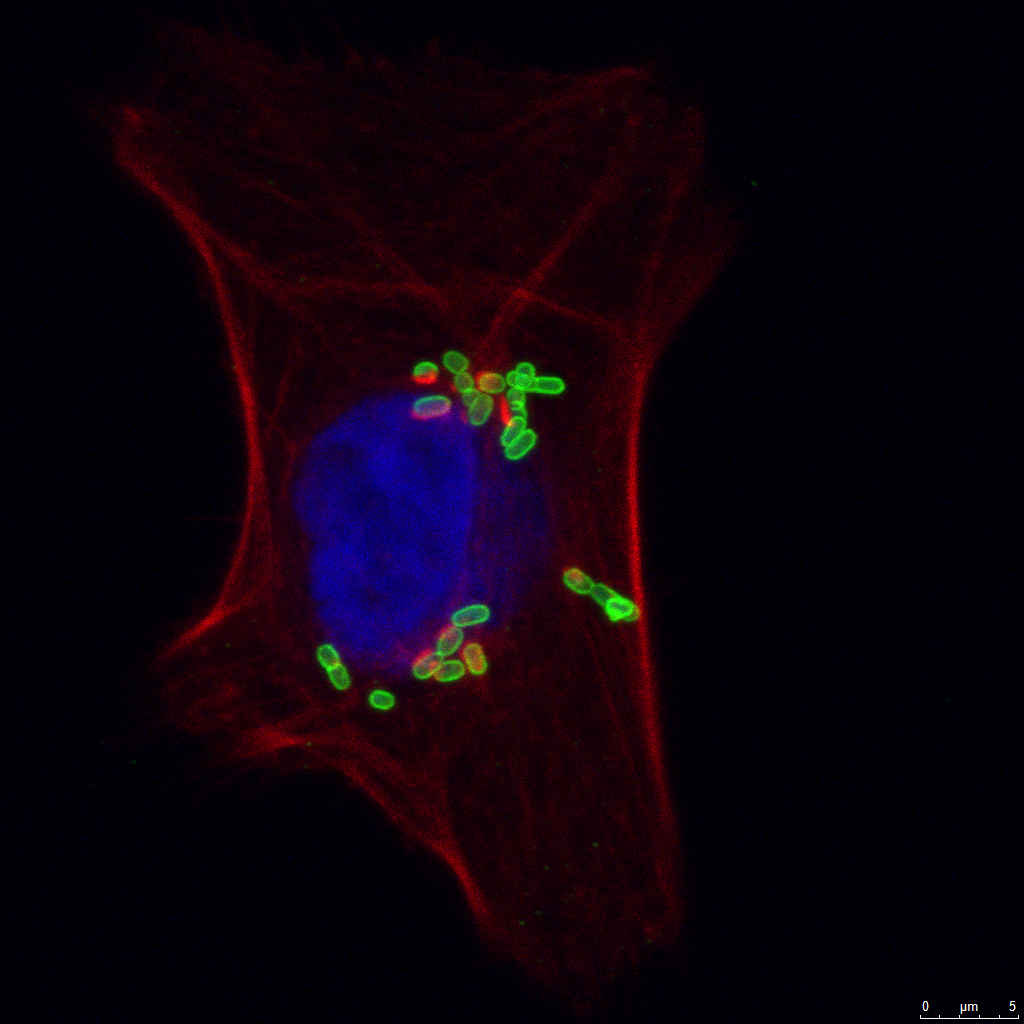
- Cellular/subcellular localization and colocalization studies.
- Tile imaging.
- Multichannel fluorescence and/or transmission imaging (BF, DIC, phase contrast).
- Multidimensional in vivo time lapse experiments (wound healing, infection, etc).
- Single, sequential or GFP/mCherry simultaneous TIRF for the study of dynamic processes or molecule localization at the cell surface level.
- Tile-scan imaging.




Name |
Position |
Contact |
| José Mª Requejo | Scientific coordinator | |
| Ana Oña Blanco | Head of Service | |
| Gianluca D´Agostino | Technician | |
| Jaime Fernández de Córdoba | Technician |