PLoS ONE
Lioy VS, Machon C, Tabone M, Gonzalez-Pastor JE, Daugelavicius R, Ayora S, Alonso JC.
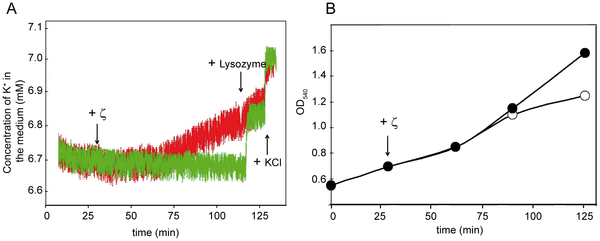
The ζε module consists of a labile antitoxin protein, ε, which in dimer
form (ε(2)) interferes with the action of the long-living monomeric ζ
phosphotransferase toxin through protein complex formation. Toxin ζ,
which inhibits cell wall biosynthesis and may be bactericide in nature,
at or near physiological concentrations induces reversible cessation of
Bacillus subtilis proliferation (protective dormancy) by targeting
essential metabolic functions followed by propidium iodide (PI) staining
in a fraction (20-30%) of the population and selects a subpopulation of
cells that exhibit non-inheritable tolerance (1-5×10(-5)). Early after
induction ζ toxin alters the expression of ∼78 genes, with the
up-regulation of relA among them. RelA contributes to enforce
toxin-induced dormancy. At later times, free active ζ decreases
synthesis of macromolecules and releases intracellular K(+).
We propose
that ζ toxin induces reversible protective dormancy and permeation to
PI, and expression of ε(2) antitoxin reverses these effects. At later
times, toxin expression is followed by death of a small fraction (∼10%)
of PI stained cells that exited earlier or did not enter into the
dormant state. Recovery from stress leads to de novo synthesis of ε(2)
antitoxin, which blocks ATP binding by ζ toxin, thereby inhibiting its
phosphotransferase activity.
